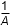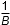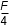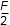Study Guide
Field 097: Physical Science
Sample Multiple-Choice Questions
Expand All Answers | Collapse All Answers
The following reference material will be available to you during the test:
Subarea 1—FOUNDATIONS OF SCIENTIFIC INQUIRY
Objective 001
Understand the principles and procedures of scientific inquiry.
1. A researcher collects data on the structure of the atom by directing alpha particles at a thin sheet of gold foil. After repeated trials, as predicted, most of the alpha particles pass through the gold foil without being significantly deflected from their path. To the surprise of the researcher, a small percentage of the alpha particles are scattered back from the gold foil. Given the small percentage of alpha particles that do not pass through the foil, which of the following should the researcher do?
- Disregard the anomalous events since they are unrepresentative of the majority of the data.
- Develop an explanation for the anomalous events and disregard the results that were predicted.
- Continue the experiment until the results match the predictions that were made by the researcher.
- Incorporate the anomalous data and the expected results into an explanation that can account for both.
- Enter to expand or collapse answer.Answer expanded
- Correct Response: D.
2. The graph below shows the relationship between two variables, A and B.
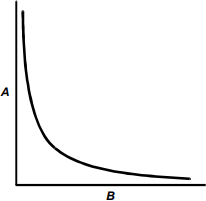
The vertical axis is labeled A, and the horizontal axis is labeled B. The data line begins high near the vertical axis, descends steeply, curves smoothly away from the origin, and gradually approaches the horizontal axis.
Which of the following is most likely to result in a straight-line graph?
- Plot 1 over A versus B.
- Plot 1 over A versus 1 over B.
- Plot A squared versus B.
- Plot B versus A.
- Enter to expand or collapse answer.Answer expanded
- Correct Response: A.
Objective 002
Apply knowledge of methods and equipment used in scientific investigations.
3. A chemist is analyzing the concentration of a particular contaminant in a water sample. After running the test repeatedly, the chemist calculates the standard deviation of the concentration data. In this situation, the standard deviation of the data is most useful for:
- evaluating the accuracy of the analysis.
- determining the cause of variability in the results.
- assessing the precision of the measurements.
- establishing the average value of the data.
- Enter to expand or collapse answer.Answer expanded
- Correct Response: C.
Objective 003
Understand the development of scientific thought and inquiry.
4. A researcher announces a major breakthrough in nuclear fusion that could make it a commercially viable energy source. After reviewing the research and evaluating the researcher's claims about fusion, scientists in the same field reject the findings. Which of the following provides an acceptable reason for the scientific community to reject the researcher's findings?
- The results do not support the claim that the breakthrough will make fusion commercially viable.
- The researcher did not publish the complete data set generated by the investigation.
- The results could not be reproduced by independent scientists using the same research procedures.
- The researcher was not well known in the field before announcing the breakthrough.
- Enter to expand or collapse answer.Answer expanded
- Correct Response: C.
Objective 004
Understand the relationships of physical science to technological and social issues, both contemporary and historical.
5. Which of the following is most important in assessing the credibility of scientific claims?
- the researcher's previous work on the same subject
- the researcher's results are reproducible
- the researcher's affiliation with a major university or institution
- the researcher's findings are consistent with earlier work
- Enter to expand or collapse answer.Answer expanded
- Correct Response: B.
Objective 005
Understand interrelationships among the physical, life, and earth/space sciences.
6. The concept of entropy is most closely associated with which of the following physical laws?
- second law of thermodynamics
- law of the conservation of energy
- Newton's law of inertia
- law of universal gravitation
- Enter to expand or collapse answer.Answer expanded
- Correct Response: A.
Subarea 2—CONCEPTS AND PRINCIPLES OF CHEMISTRY
Objective 006
Understand chemical properties of matter.
7. Use the structural formula for lactic acid below to answer the question that follows.
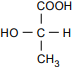
The structural formula has C at the center. COOH is bonded above the C, with the bond at the C. HO is bonded to the left, with the bond at the O. CH3 is bonded below, with the bond at the C. H is bonded to the right.
Which of the following is the optical isomer for the lactic acid molecule shown above?
-
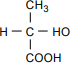 The structural formula has C at the center. CH3 is bonded above the C, with the bond at the C. H is bonded to the left. COOH is bonded below, with the bond at the C. HO is bonded to the right, with the bond at the H.
The structural formula has C at the center. CH3 is bonded above the C, with the bond at the C. H is bonded to the left. COOH is bonded below, with the bond at the C. HO is bonded to the right, with the bond at the H.
-
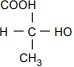 The structural formula has C at the center. COOH is bonded above the C, with the bond at the H. H is bonded to the left. CH3 is bonded below, with the bond at the H3. HO is bonded to the right, with the bond at the H.
The structural formula has C at the center. COOH is bonded above the C, with the bond at the H. H is bonded to the left. CH3 is bonded below, with the bond at the H3. HO is bonded to the right, with the bond at the H.
-
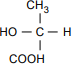 The structural formula has C at the center. CH3 is bonded above the C, with the bond at the H3. HO is bonded to the left, with the bond at the O. COOH is bonded below, with the bond at the H. H is bonded to the right.
The structural formula has C at the center. CH3 is bonded above the C, with the bond at the H3. HO is bonded to the left, with the bond at the O. COOH is bonded below, with the bond at the H. H is bonded to the right.
-
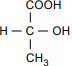 The structural formula has C at the center. COOH is bonded above the C, with the bond at the C. H is bonded to the left. CH3 is bonded below, with the bond at the C. OH is bonded to the right, with the bond at the O.
The structural formula has C at the center. COOH is bonded above the C, with the bond at the C. H is bonded to the left. CH3 is bonded below, with the bond at the C. OH is bonded to the right, with the bond at the O.
- Enter to expand or collapse answer.Answer expanded
- Correct Response: D.
Objective 007
Understand the physical properties of matter.
8. Use the phase diagram below to answer the question that follows.
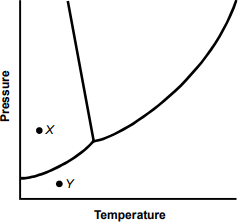
The phase diagram is a graph with the vertical axis labeled Pressure and the horizontal axis labeled Temperature. The data line begins low on the vertical axis, curves gradually upward, then splits. One branch ascends in a straight line that slants slightly back toward the vertical axis. The other branch ascends in a shallow curve at about a 45° angle to the right. There are two points, labeled X and Y. Point X lies above the initial data line, between the vertical axis and the left branch, slightly above the level of the split. Point Y lies below the initial data line, about halfway between the vertical axis and the split, somewhat further to the right than point X.
In the phase diagram for water shown above, X and Y represent different pressure and temperature conditions. Which of the following processes occurs as water makes the transition from the pressure and temperature conditions at point X to the conditions at point Y?
- liquefaction
- sublimation
- evaporation
- condensation
- Enter to expand or collapse answer.Answer expanded
- Correct Response: B.
Objective 008
Understand the properties and characteristics of chemical bonds.
9. Water does not break down into its constituent atoms until its temperature is raised to approximately 2,700 degrees C. Which of the following is responsible for the great stability of water molecules?
- high heat capacity
- strong covalent bonds
- low chemical reactivity
- flexible hydrogen bonds
- Enter to expand or collapse answer.Answer expanded
- Correct Response: B.
Objective 009
Understand the types and characteristics of chemical reactions.
10. Use the information below to answer the question that follows.
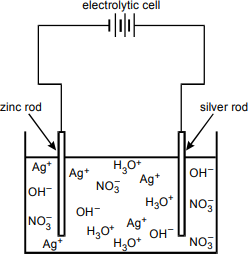
The diagram shows an open container of liquid. The liquid is represented by multiple symbols of AG+, O H negative, N O3 negative, and H3O+. A zinc rod is connected to one terminal of an electrolytic cell, and a silver rod is connected to the other terminal. The free end of each rod stands partially in the liquid.
Two rods, one made of silver and one of zinc, are placed in a solution of silver nitrate, as shown in the diagram above. Which of the following is primarily responsible for the increase in the mass of the zinc rod?
- the changing concentration of silver ions in solution caused by the oxidation of the silver rod due to the flow of electrons through the circuit
- the changing mass of the silver nitrate solution caused by the oxidation of the nitrate ions and silver ions in solution
- the reduction of silver ions in solution that are then deposited on the zinc rod due to the flow of electrons in the circuit
- the oxidation of zinc ions at the zinc rod caused by the different reduction potentials of the zinc ions and silver ions in solution
- Enter to expand or collapse answer.Answer expanded
- Correct Response: C.
Objective 010
Apply the principles and methods of stoichiometry and the rules of chemical nomenclature and notation for inorganic and organic substances.
11. Use the balanced chemical equation below to answer the question that follows.
NaHCO3(s) + HCl(aq) right arrow NaCl(s) + H2O(aq) + CO2(g)
A scientist puts 4.20 g of NaHCO3 into an evaporating dish and adds dilute HCl until the production of a gas ceases. The scientist then slowly evaporates water from the evaporating dish and recovers 3.04 g of NaCl. The reaction is depicted in the chemical equation shown above. What is the experimental value for the mole ratio of sodium chloride to sodium bicarbonate?
- 1.00 to 1
- 1.04 to 1
- 1.38 to 1
- 1.44 to 1
- Enter to expand or collapse answer.Answer expanded
- Correct Response: B.
Objective 011
Understand analytical techniques.
12. Which of the following provides the best example of qualitative analysis?
- identifying the number of moles of a particular compound in a sample
- determining the ratio of two specific elements in a compound
- identifying the different types of ions present in a sample
- determining the average density of a mixture
- Enter to expand or collapse answer.Answer expanded
- Correct Response: C.
Subarea 3—CONCEPTS AND PRINCIPLES OF PHYSICS
Objective 012
Analyze forces and motion in one and two dimensions.
13. Which of the following describes a difference between the concepts of mass and weight?
- Weight provides a measure of the force acting on an object, while mass does not.
- Weight takes volume into account, while mass does not.
- Mass takes the gravitational force acting on an object into account, while weight does not.
- Mass provides a measure of density, while weight does not.
- Enter to expand or collapse answer.Answer expanded
- Correct Response: A.
14. The buoyant force exerted by a fluid on an entirely submerged object is equal to:
- the mass of the submerged object.
- the gravitational force acting on the submerged object.
- the density of the submerged object.
- the weight of the fluid displaced by the submerged object.
- Enter to expand or collapse answer.Answer expanded
- Correct Response: D.
Objective 013
Understand conservation laws and thermodynamics.
15. Which of the following best describes the concept of temperature?
- the thermal energy transferred between objects as a result of conduction
- the total internal energy contained in the atoms or molecules composing a substance
- the total heat energy contained within an object or substance per unit volume
- the average kinetic energy of the atoms or molecules composing a substance
- Enter to expand or collapse answer.Answer expanded
- Correct Response: D.
Objective 014
Understand the characteristics of waves and wave motion, including the principles of sound and acoustics.
16. A child is standing at a bus stop on the side of the road when a fire truck goes by at a constant speed sounding its alarm. The sound heard by the child rises in pitch as the fire truck approaches and then falls in pitch after the fire truck passes and travels away from the child. Which of the following best explains the change in pitch heard by the child?
- The speed of the sound generated by the truck's alarm changes due to the change in the direction of motion of the sound source relative to the child.
- The energy of the sound waves being produced by the truck's alarm increases and then decreases as the truck approaches and then moves away from the child.
- The amplitude of the sound generated by the truck's alarm increases and then decreases inversely with the change in its position relative to the child.
- The perceived frequency of the sound waves generated by the truck's alarm is altered due to the change in its direction of movement relative to the child.
- Enter to expand or collapse answer.Answer expanded
- Correct Response: D.
Objective 015
Understand basic principles of electromagnetism.
17. Use the circuit diagram below to answer the question that follows.
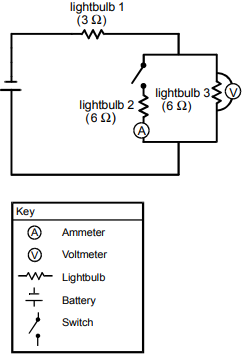
The diagram shows a switch, lightbulb #2, an ammeter, and lightbulb #3 in a circuit. Both lightbulb #2 and lightbulb #3 are labeled as 6 ohm. A voltmeter is wired to either side of lightbulb #3. A wire from a point between the switch and the voltmeter goes to lightbulb #1, then to a battery, then to a point between the ammeter and the voltmeter. Lightbulb #1 is labeled as 3 ohm.
The circuit diagram shown above includes three lightbulbs, a voltmeter, an ammeter, and a switch. Which of the following will occur if the switch is closed?
- The internal resistance of the battery will increase.
- The brightness of lightbulb 1 will increase.
- The current through lightbulb 3 will increase.
- The electric potential measured across lightbulb 3 will increase.
- Enter to expand or collapse answer.Answer expanded
- Correct Response: B.
18. Use the information below to answer the question that follows.
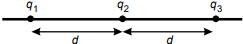
In the diagram above, three identical point charges are located on a straight line and are separated by the same distance, d. If F equals the magnitude of the force acting on q1 due to q2, what is the magnitude of the force on q1 due to q3?
- F over 4
- F over 2
- 2F
- 4F
- Enter to expand or collapse answer.Answer expanded
- Correct Response: A.
Objective 016
Understand the electromagnetic spectrum and the properties of electromagnetic waves.
19. Which of the following technologies relies on energy changes of the electrons in the innermost orbital of an atom?
- fluorescent lighting
- X-ray photography
- magnetic resonance imaging
- microwave ovens
- Enter to expand or collapse answer.Answer expanded
- Correct Response: B.
Objective 017
Understand the basic concepts and applications of modern physics.
20. During the nineteenth century, researchers demonstrated that shining a light on various metals caused electrons to be released from the surface of the metal. Which of the following best explains this phenomenon?
- A photon striking the metal's sur-face is absorbed by the electron it collides with, ejecting the electron from the surface of the metal.
- A light wave striking the metal's surface increases the thermal energy of the metal atom, causing it to eject valence electrons from the highest energy orbital.
- Light waves striking the surface of the metal slowly increase the vibration of electrons on the surface until some are able to break free.
- Photons repeatedly striking atoms at the metal's surface transfer kinetic energy to electrons until the electrons gain enough energy to escape from the surface.
- Enter to expand or collapse answer.Answer expanded
- Correct Response: A.